Allowing biologists to study subcellular activity in finer detail than ever before
Eric Betzig was awarded the Nobel Prize in Chemistry, along with Stefan W. Hell and William E. Moerner, for the development of the Super-Resolved Fluorescence Microscopy technique, which reveals high-resolution 3D images of subcellular structure, allowing biologists to study the activity of molecules, cells and embryos in finer detail than ever before.
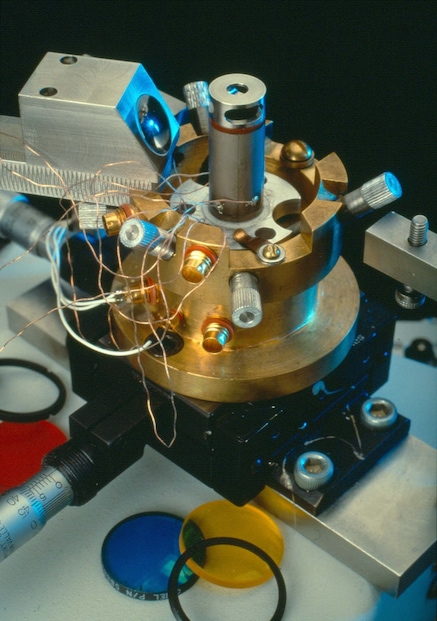
Betzig joined Bell Labs in Murray Hill in 1988 and worked to improve the Near-Field Scanning Optical Microscopy (NSOM) technology he had helped develop at Cornell during his PhD studies. Betzig collaborated with Jay Trautman, Tim Harris and others to refine the NSOM technology and try out new applications. They achieved a resolution of 12 nanometers with their near-field probe, far better than the diffraction limit. The probe also yielded signals more than 10,000 times larger than those reported previously.
The first to break the diffraction barrier, the powerful microscope was seen as a new means to inspect integrated circuits and examine cells. Collaborations with Trautman, Harris, Harald Hess and Rob Chichester allowed Betzig to explore several more applications for the NSOM technology, including high-density data storage, semiconductor spectroscopy and super-resolution fluorescence cell imaging.
The exploration yielded key breakthroughs that brought Betzig closer to his dream of imaging molecules within living cells, with several breakthroughs along the way including:
- A new high-density magneto-optic storage technique, supporting data densities of 45 billion bits per square inch — nearly 100 times the density provided by the best commercial magneto-optic methods of the day (Betzig and Trautman, 1992)
- The first single molecule microscopy at room temperature and the first determination of single molecule dipole orientations (Betzig and Chichester, 1993)
- Near-field technology with low temperature scanning to observe luminescent centers in semiconductors with high spatial resolution (Betzig and Hess, 1994).
Betzig and Hess continue to collaborate at the Janelia Research Campus of the Howard Hughes Medical Institute (HHMI). Betzig left Bell Labs in 1994 to work with his father in machine tool design, but after a decade returned to research and resumed collaboration with Hess, building on the groundbreaking work they had done together at Bell Labs. They built a super-resolution microscope that formed the basis for photo-activated localization microscopy (PALM) technology in 2006. The PALM technology allowed Betzig and Hess to optically image intracellular proteins at nanometer spatial resolution.