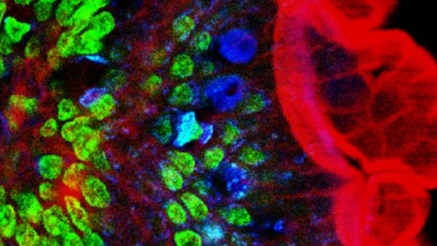
Several powerful technologies for imaging the functions of living cells were developed or refined at Bell Labs in the Biological Computation Research area during the 1990s, including two-photon microscopy [1], functional MRI [2] and near-field optical microscopy—which later led to super-resolution optical microscopy [3].
The role of physics in neuroscience
Although we have known of the existence of neurons for over a century, we do not yet understand the brain completely. Only in the last few years have we begun to develop instruments capable of measuring the microscopic chemical and electrical processes that take place in brain tissue. We are beginning to correlate these processes with high-level functions such as memory, learning and neural diseases.
Functional imaging
The imaging techniques developed at Bell Labs have evolved to be dominant underpinnings to the growth of fundamental neuroscience, allowing researchers to examine the function of individual nerve cells with high precision, especially how nerve cells communicate with each other in networks. This is a huge step forward in the understanding of the physical mechanisms of the human brain and in the understanding of how the brain’s networks process information. Researchers have also been able to follow how connections between nerve cells are established in the developing brain.
Functional imaging has led to identification of signaling pathways that control communication between nerve cells and provide the basis for memory, and it has enabled the study of nerve cell activity in those networks that controls vision, hearing and movement.
Two-photon microscopy explained
Two photons can combine to be absorbed as a single photon having the sum of the individual photon energies. The process was originally conceived in 1931 by Maria Goeppert-Mayer in 1931 [4], but experimental verification required the development of the laser to provide a sufficiently intense coherent light source.
Fundamental to two-photon microscopy is the excitation of a molecule by two photons through a virtual state. This can only happen when the optical field is sufficiently intense that two photons can be present at the same time. The molecule is excited by an amount of energy equal to the sum of the individual photons (e.g., two times one photon in this case). The molecule relaxes and emits a single photon with energy equal to the difference between the excited state and the ground state (energy gap).
In two-photon microscopy, a pulsed beam of infrared photons is focused into a sample and the molecules that have exactly the right absorption energy gap can absorb the doubled photons. This is a nonlinear process that depends very strongly on the light intensity, and therefore depends strongly on the way in which the light is focused. A detector tuned to the emission energy (two times the exciting photon energy) will measure light from those molecules that have been excited. The source and detector are aimed (scanned) back and forth across the sample to form an excitation image that is a picture of the proteins of interest.
With the development of advanced lasers and scanning techniques, Winfried Denk conducted the world's first two-photon microscopy measurements during his postdoctoral work at Cornell [5], and he refined the technique at Bell Labs with David Tank, and later with Karel Svoboda [6].
Denk describes several factors that make two-photon imaging with infrared photons attractive [7].
Optical scattering strongly increases with shorter photon wavelengths, so that visible excitation (and spectroscopy) of structures more than a few tens of micrometers deep is impractical. Infrared photons can penetrate considerably deeper (approximately hundreds of micrometers) before scattering is prohibitive. Because the signal is only generated when 2 photons are absorbed, single photon background noise is significantly reduced at the wavelength of interest. The use of low energy photons, plus ultra-short laser pulses, keeps the phototoxicity effects low, allowing extended study of living cells.
Future of multiphoton imaging
Today’s neuroscientists often turn to multiphoton excited fluorescence microscopy (MPEF) for in vivo imaging [8]. With thousands of MPEF microscopes in use worldwide and improvements in laser systems, optics and fluorescence proteins, we are seeing improvements in depth, resolution and sensitivity. Researchers have demonstrated the ability to study a single cell over an extended time – for example watching the growth of an embryo over several hours [8].
Key researchers
David Tank
David Tank is the Henry L Hillman Professor in Molecular Biology and the co-director of the Princeton Neuroscience Institute. He received his PhD from Cornell University in 1983 and spent the next 18 years at Bell Labs, first as a post-doc, then as a Member of Technical Staff (MTS), then a Distinguished MTS in 1988. He led the Biological Computation Research department (Biophysics) from 1991 to 2001. During this time he worked with Seiji Ogawa on functional MRI and with Winfried Denk and Karel Svoboda on two-photon microscopy. [9]
Winfried Denk
Winfried Denk performed the first two-photon microscopy measurements at Cornell with Watt W Webb. He further developed and applied the technique to living cells during his tenure at Bell Labs from 1991 to 1999. He moved to the Max Planck Institute in 1999. Denk is the director of the Max Planck Institute of Neurobiology in Martinsried.
Karel Svoboda
Karel Svoboda is a group leader at the HHMI Janelia Research campus, with research focused on methods of revealing details of neural functions. He received his PhD from Harvard in biophysics and did postdoctoral research at Bell Labs with Denk and Tank.
Recognition
The Grete Lundbeck European Brain Research Prize, commonly known as The Brain Prize, recognizes highly original and influential advances in any area of neuroscience.
The 2015 Brain Prize award honors work by David Tank, Winfried Denk, Karel Svoboda (during their tenure at Bell Labs) and Arthur Konnerth which resulted in commercially available two-photon microscopes that can be used to look at how brain processes work at a molecular level.
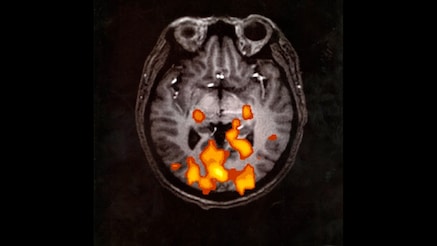
1990: A process developed at Bell Labs shows (in color) mental activity in the brain at the time of the MRI scan. In this case the subject was looking at flashing light.
References
[1] W Denk , JH Strickler, and WW Webb, “Two-photon laser scanning fluorescence microscopy,” Science. 1990 Apr 6, 248 (4951) 73-76.
[2] S Ogawa, TM Lee, AR Kay, and DW Tank, “Brain magnetic resonance imaging with contrast dependent on blood oxygenation,” PNAS 1990 87 (24) 9868-9872.
[3] E Betzig and RJ Chichester, “Single molecules observed by near-field scanning optical microscopy,” Science 262, 1422 (1993).
[4] Goeppert-Mayer M (1931). “Über Elementarakte mit zwei Quantensprüngen,” Annals of Physics 9(3), 273–95.
[5] W Denk, J Strickler, W Webb, “Two-photon laser scanning fluorescence microscopy,” Science 1990, 248 (4951).
[6] W Denk, Svoboda, “Photon upmanship: why multiphoton imaging is more than a gimmick,” Neuron 1997 18 (3), 351-357.
[7] W Denk, KR Delaney, A Gelperin, D Kleinfeld, BW Strowbridge, DW Tank, R Yuste, “Anatomical and functional imaging of neurons using 2-photon laser scanning microscopmy,” Journal of Neuroscience Methods 54 (1994) 151-162.
[8] J Klein and P.G Smith, “Multiphoton Imaging Takes New Directions,” Biophotonics, March 2015.
[9] DW Tank CV is available at https://pni.princeton.edu.